October 24, 2018 NeuWave Medical, Inc. Dan Kosednar Director of Regulatory Affairs and Quality Assurance 3529 Anderson Street Ma

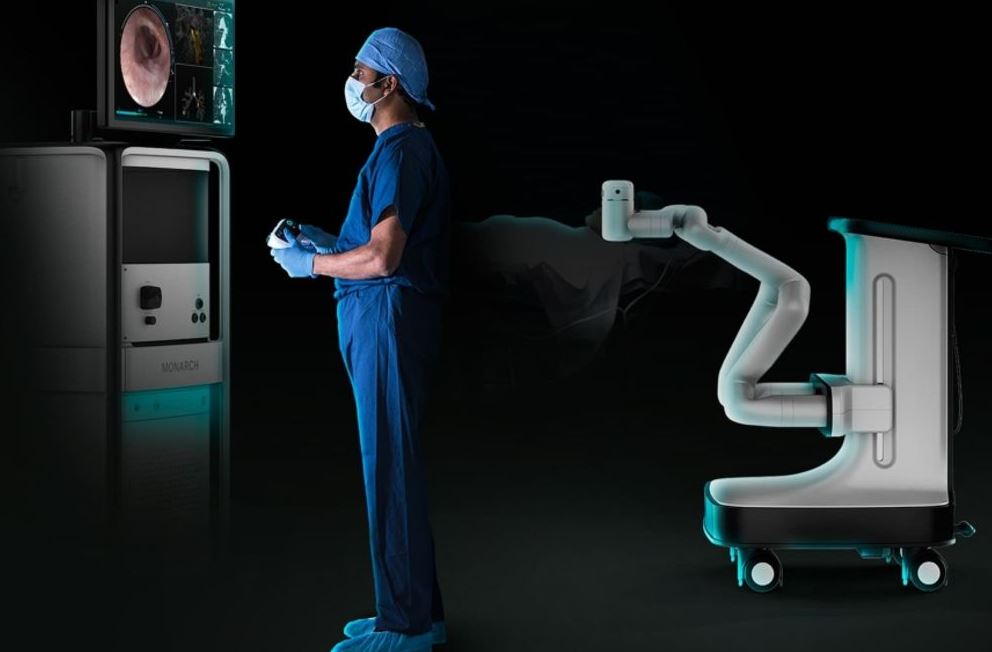
Interventional pulmonology suite with equipment arrangement showing... | Download Scientific Diagram
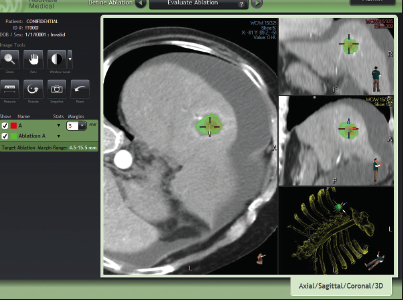
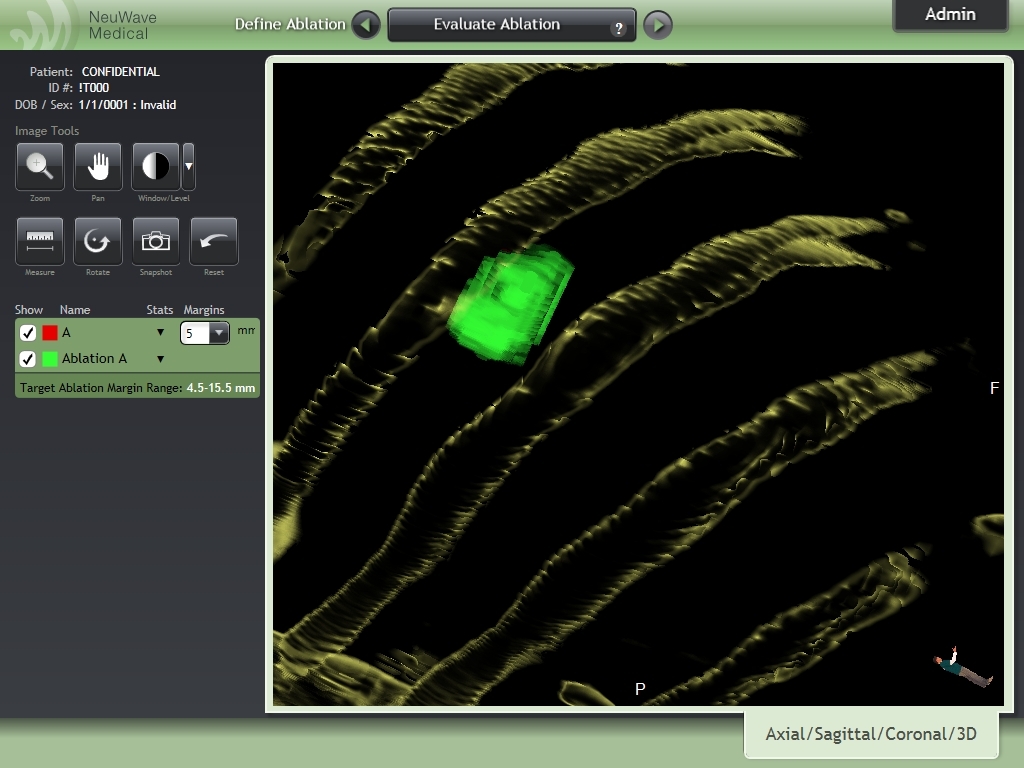
NeuWave Collaboration: Shared Values Lead to Improved Patient Care – Department of Radiology – UW–Madison